Theory of Lewis acid zeolite catalysis for biomass conversion


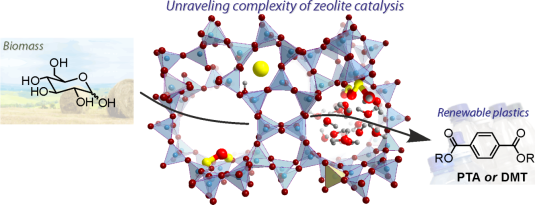
Terephtalic acid (PTA) is an industrially important chemical used for the production of polyethylene terephthalate (PET), the polymer widely used for the manufacture of various synthetic fibers, composite materials and food/beverages packaging. Currently, PET is produced by oxidation of oil-derived p-xylene. The development of efficient green routes to products such as PET by using biomass as feedstock is crucial to meet the sustainability goals of the chemical industry. Zeolites are important catalysts in the upgrading of biomass to green precursors for renewable PET production. Lewis acid sites in zeolites are able to isomerize and dehydrate glucose, the main sugar component in lignocellulosic biomass, into hydroxymethylfurfural and also its subsequent conversion to desired aromatics. This route is relevant for tailored production of aromatics from sugar streams but may also play a role in catalytic pyrolysis upgrading schemes. In this project the researchers will investigate by sophisticated quantum-chemical methodologies the key aspects that control optimum reactivity of Lewis acid zeolites for upgrading of biomass. Although especially Brønsted acid catalyzed conversion in zeolites has been extensively studied, reactivity concepts of Lewis acidity in the presence of a solvent are much less developed. This project will develop such concepts with the aim to rationalize the way Lewis acid zeolites catalyze conversion of sugars and furans and provide clues how to design optimal catalysts for such conversion steps.
Project leader: Prof. Emiel Hensen