Catalysis: Storing Green Energy for the Future
Project description
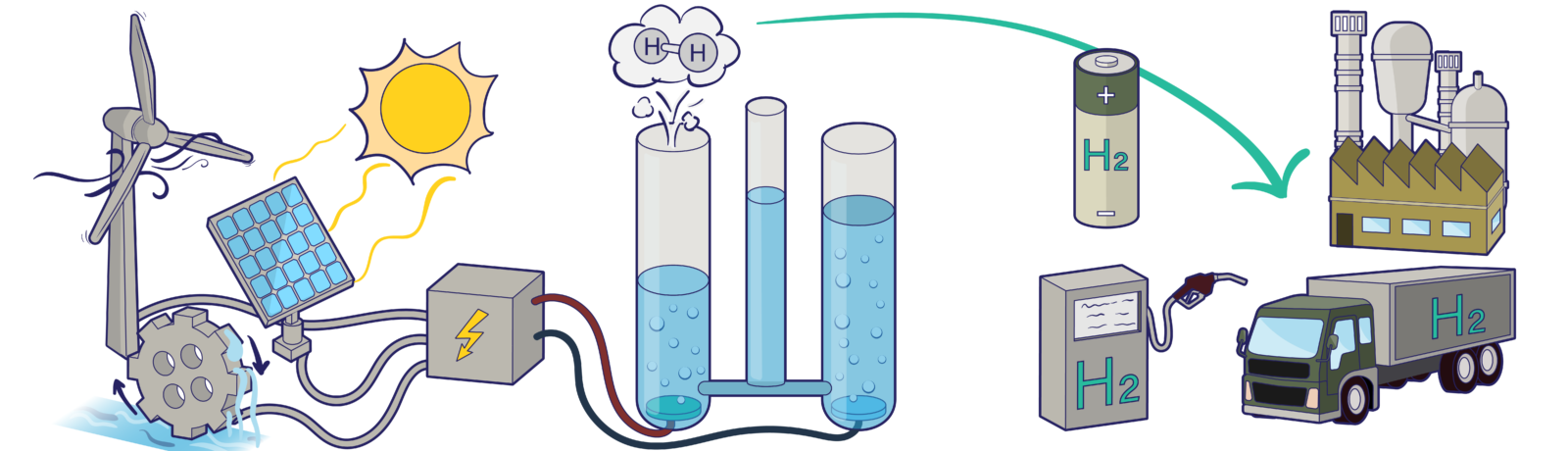
We use energy every day and we want clean, plentiful and reliable sources that do not pollute our only planet. Electrons generated from various clean energy sources such as solar and wind can be converted into other forms of energy. We can store this energy in the chemical bonds in molecules such as hydrogen. We demonstrate the fundamentals behind this transformation in a fun, visually engaging way by bringing the whole process to your table top. With our demonstrator you can see your own work generating clean hydrogen right before your eyes!
Advanced summary
Our demonstrator is a simple glass apparatus with openings for inserting electrodes. The electrodes can be powered using different power sources such as solar panels, a water turbine, and a crank shaft that participants can drive manually.
Energy conversions happen right from the power sources. The solar panel converts light to electrical energy, the wind turbine and the crankshaft convert mechanical energy to electrical energy. Electrical energy is transported by the movement of electrons through the wires. The energy of these electrons splits water molecules into hydrogen and oxygen gas at the electrodes. This too is an energy conversion! This time, from electrical energy to chemical energy. Hydrogen gas is therefore, one way to store energy from renewable sources. We can later use the energy stored in these chemical bonds to power our homes and cars! The audience gets to see the latter in action as we run a small hydrogen driven toy car right in front of their eyes.
The learning outcomes can be tailored for audiences from a high school level to a research level. Some concepts that can be taught using the demonstrator are: basic redox chemistry, the nature of current and voltage, and basics of electrochemical conversions. Besides this, the setup helps demonstrate the possibility of storing and using clean energy using hydrogen as an energy carrier. For more advanced audiences, it is possible to help visualize concepts in heterogenous catalysis using 3D printed models and some cool videos of molecules in action on catalyst surfaces!