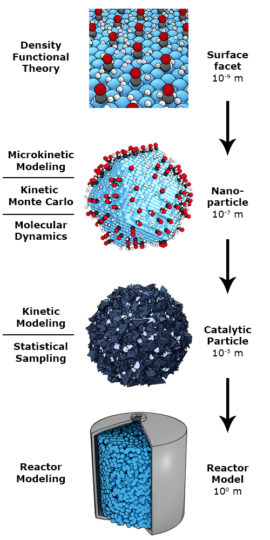
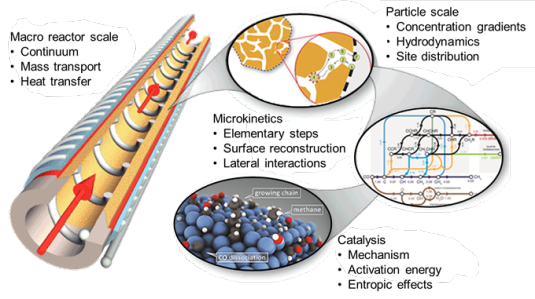
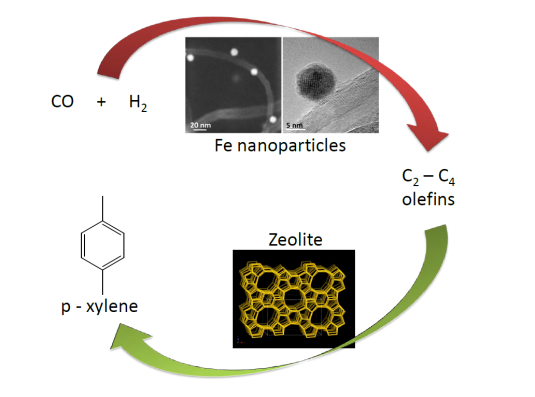
The rational design of next-generation catalysts that will contribute to solving the impending energy and environmental challenges requires accurate description of mesoscale phenomena in catalysis. Current state-of-the-art modelling techniques mostly focus either on the nanoscale description of individual elementary reaction steps or on the macroscale to describe behavior of reactors, usually employing lumped reaction kinetics. In this project, new modelling tools are developed to study emergent phenomena at the mesoscale that lead to evolution of the catalyst structure as a result of changes in the surface adsorbed layer. In detail, we investigate the complex processes occurring in the Fischer-Tropsch reaction, an industrially important reaction for the synthesis of transport fuels and chemicals. In this reaction, many important details remain unclear: the influence of lateral interactions, surface reconstruction under catalytic conditions, migration of adsorbates between different surface facets of nanoparticles and deactivation due to strongly adsorbing reaction intermediates are far from understood. Describing these mesoscale phenomena with sufficient accuracy leads to opportunities to guide the design of novel improved catalysts.
Project leader: Dr. Ivo Filot